Identify the Type of Hydrocarbon Represented by Each Structure.
If the compound represents two or more functional groups list all groups being represented. Select the correct answer below.

Hydrocarbon Summary Britannica
Hydrocarbon structures and types of isomerism structural isomers cistrans isomers and enantiomers.

. These compounds are called hydrocarbons An organic compound composed of carbon and hydrogen. It is not a saturated fatty acid because saturated fatty acids do not have any double bonds between the carbons in the hydrocarbon chain. A two-carbon chain is called ethane.
This is the simplest member of a series of hydrocarbons. Hydrocarbon structures and isomers. Aliphatic hydrocarbons A hydrocarbon based on chains of C atoms.
And a four-carbon chain butane. This is the currently selected item. Unsaturated fatty acids have at least one double bond between the carbons in the hydrocarbon chain.
Each successive member of the series has one more Carbon atom than the preceding member. Option B we have a kind. Hydrocarbons are compounds which contain only carbon and hydrogen.
Due to their different molecular structures the empirical formula of hydrocarbons is also different from each other. This leads to differences in geometries and in the hybridization of the carbon orbitals. Alkane Alkyne Alkyne Choose.
The carbons atoms can be Sp Sp 2 or Sp 3 hybridised. In this case there is triple born between the carbon atoms auction e We have saturated hydrocarbon which contain a maximum number off hydrogen Adam link for carbon atom. Alkanes or saturated hydrocarbons contain only.
Identify the functional group each compound represents. O Br CH3 CH3 H H H CH3 CH3 Br. This series of compounds are called alkanes CnH2n2.
Alkane alkene O alkyne O aromatic. Write your answer on the space provided below each item. Assign the suffix based on the type of carbon -carbon bonds.
Experts are tested by Chegg as specialists in their subject area. If the compound is a hydrocarbon write the specific type of hydrocarbon class it belongs. Guidelines for Naming Branched-Chain Hydrocarbons.
A kind of coal-based jet fuel was made out of coal direct liquefied oilBased on GC-MS analysisits hydrocarbon group-type consists of 32 monocyclo-alkane423 bicyclo-alkane136 tricycle. Br CH CH3 Br Н Br CH3. Identify the functional group each compound represents.
E- At normal temperatures and. C- The CC bond is their functional group. MAINIdeaSome hydrocarbons have the same molecular formula but have different molecular structures.
D- The boiling points of alkenes increase with relative molecular mass. MAINIdeaAlkenes are hydrocarbons that contain at least one double bond and alkynes are hydrocarbons that contain at least one triple bond. Find the longest continuous C -chain and assign the root name.
Identify the type of hydrocarbon represented by each structure. Each dash - represents a single covalent bond in the structural formula where two atoms share one pair of valence electrons. Straight-chain alkanes can be named by attaching a prefix indicating the number of carbon atoms to the suffix -ane A displayed formula is a pictorial representation of a molecule where lines are drawn to represent the bonds between each atom.
Write your answer on the space provided below each item. Hydrocarbons themselves are separated into two types. Saturated hydrocarbons have only single bonds between carbon and hydrogen atoms and are called alkanes.
There is a type of hydrocarbon in which the carbon atoms are connected to each other to form a chain. Choose Consider the reaction of bromine with the pictured akene. Double triple bonds are assigned the lowest possible number.
Are hydrocarbons based on chains of C atoms. These prefixes can be seen in the names of the alkanes described in Table 1. There are three types of aliphatic hydrocarbons.
A- Alkenes are insoluble in non-polar solvents such as cyclohexane. B- Alkenes are unsaturated hydrocarbons. - Bra CHE Which structure shows the correct bonding for the product.
Identify the type of hydrocarbon represented by each structure. Image will be Uploaded Soon Types of Saturated Hydrocarbons. Solution for Which type of hydrocarbon is represented by this structure.
Hydrocarbons can further be classified as alkanes alkenes or alkynes. A three-carbon chain propane. Assign a locator number to all substituents.
We review their content and use your feedback to keep the quality high. See we have l keen In this case the atoms have double born with each other. The lighter ones are gases and used as fuels.
If the compound is a hydrocarbon write the specific type of hydrocarbon class it belongs. The structure represents a fatty acids with an omega 6 double bond between the carbons in the hydrocarbon chain. Contain only Carbon single bonds.
Longer chains are named as follows. If the compound represents two or more functional groups list all groups being represented. Contain at least one Carbon triple bond.
Aliphatic hydrocarbons and aromatic hydrocarbons. But the Adam is in sai click structure options. Contain at least one Carbon double bond.
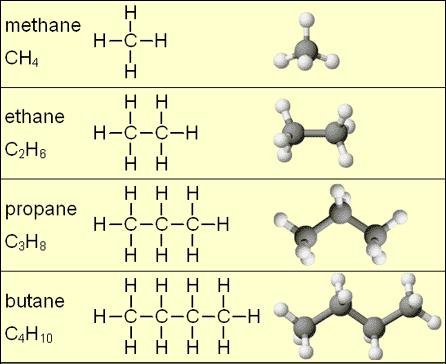
The simplest Hydrocarbon is methane CH4. Here weve taken an example of the general saturated hydrocarbon formula for Ethane C 2 H 6 where the structure is represented below. Write the Lewis structure for C3Hg.
They are hydrocarbons having a ring structure in them. We can distinguish several types of hydrocarbons by differences in the bonding between carbon atoms. Pentane five-carbon chain hexane 6 heptane 7 octane 8 nonane 9 and decane 10.
Hydrocarbons are organic compounds that are made of only hydrogen and carbon atoms.

Hydrocarbon Summary Britannica

Methane Molecule 3d Model Molecule Model 3d Model Methane

Coschemistry Lesson 6 05 Naming Alkanes Practices Worksheets Chemistry Worksheets Worksheet Template

Cubane C8h8 Is A Synthetic Hydrocarbon Molecule That Consists Of Eight Carbon Atoms Arranged At The Corners Of A Organic Chemistry Chemistry Book Quotes

Hydrocarbons And Its Classification Aliphatic Alicyclic And Aromatic Hydrocarbons Examples You Organic Chemistry Study Organic Chemistry Teaching Biology

How Esters Are Formed A Plus Topper Teaching Chemistry Chemistry Ester

Naming Hydrocarbons Chemistry Worksheets Chemistry Lessons Study Notes

Alkanes Vs Alkenes Vs Alkynes Chemistry Lessons Chemistry Worksheets Chemistry Basics

Tetryonics 108 02 Free Hydrocarbon Hc Ch Compounds Attach To The Unoccupied R Bond Positions Of Amino Acids H2nch R Cooh To Form The Side Chains Of All

Sat Chemistry Carbon And Organic Chemistry Hydrocarbons 299 2 Organic Chemistry Study Chemistry Organic Chemistry

Twinkle Toes Engineering Biochemistry Biology Notes Chlorophyll

Sat Chemistry Carbon And Organic Chemistry Hydrocarbons 297 1 Organic Chemistry Chemistry Carbon

Chemicaloftheday Toluene Aromatic Hydrocarbon Chemical Properties Http Bit Ly 1ybwjei Toluene Infographic Http Chemical Property Toluene Chemical

Hydrocarbon Google Search Collage Online Photoshop Online Chemical Structure

On The Basis Of The Functional Groups Listed In Chegg Com Functional Group Homework Help Function

Hydrocarbons Advanced Scribble Notes Teaching Chemistry High School Chemistry Organic Chemistry Study

Do You Know Hydrocarbon Prefixes And Suffixes In Organic Chemistry Prefixes Prefixes And Suffixes Organic Chemistry

Sat Chemistry Carbon And Organic Chemistry Hydrocarbon Derivatives The Simplest Alcohols Are Alkanes That Have One Or M Organic Chemistry Chemistry Carbon
Comments
Post a Comment